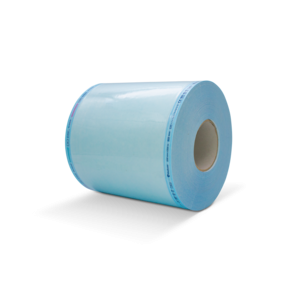
Sterilisation rolls Safeseal® Quattro

Medical device : Class I
Ref. 98*0
-
Dental -
Medical -
Industry -
Hygiene
FEATURES AND BENEFITS
TECHNICAL SPECIFICATIONS
PRODUCT INFORMATION
CERTIFICATIONS & STANDARDS
DOCUMENTS
Features and benefits
- For use as sterile barrier packaging for terminally sterilized medical devices in sterilization by steam, ethylene oxide gas in healthcare establishments
- Non-tearing, thick multi-layer film made of a combination of polypropylene and polyethylene terphthalates
- Transparent, blue tinted film facilitates both easy instrument viewing and identification of puncture or tears in the film
- Clean, fibre-free separation of paper and film ensures safe presentation of sterile products
- Permit to keep the sterilization conditions of the contained devices after sterilization: up to 1 year
Technical specifications
- Material : Heavy weight medical paper
- Weight (g/m²) : 60 g/m2
- Colors : Bleu translucide
- Model : Flat
- Clothing manufacture : Heat sealing
- Size / Dimensions : 200 m x 250 mm, 200 m x 300 mm, 200 m x 50 mm, 200 m x 75 mm, 200 m x 100 mm, 200 m x 150 mm, 200 m x 200 mm
- Process indicator : Internal/External indicators
- Sterilization method : Steam and ethylene oxide (SO)
- Made in : Taiwan
Product information
Product features
| Article | Size | Color |
|---|---|---|
| 98100 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 98110 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 9850 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 9860 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 9870 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 9880 | 200 m x 250 mm |  Translucent blue Translucent blue |
| 9890 | 200 m x 250 mm |  Translucent blue Translucent blue |
Packaging Features
| Inner case | Packaging |
|---|---|
| Rolls | Units |
| Rolls | Units |
| Rolls | 8 x Units |
| Rolls | 4 x Units |
| Rolls | 3 x Units |
| Rolls | 2 x Units |
| Rolls | Units |
Certifications & standards
- ISO 13485 Manufacturing site
- Compliant to EU Regulation 2017/745 on Medical Devices
- EN 868-5
- EN ISO 11607-1